Amyloids
Aggregation of protein has been implicated in various neurodegenerative diseases, which mainly includes Alzheimer’s disease (AD), Parkinson’s disease (PD), Multiple system atrophy (MSA) and amyotrophic lateral sclerosis (ALS). The goal of our research is to understand the process of formation of protein aggregates. We study the structural and morphological changes in the protein during aggregation process. We are interested in addressing the correlation between aggregates structure and toxicity. Measurement of the neuronal loss (symptom of neurodegenerative diseases) in mammalian models gives the information about the toxicity of aggregates.
We use several techniques to study the aggregation mechanism, including UV-visible fluorescence spectroscopy, Dynamic light scattering (DLS), Multi-angle light scattering (MALS), Circular dichroism (CD), Electron microscope (EM), Fourier transform infrared spectroscopy (FTIR), Atomic force microscopy (AFM) and Nuclear magnetic resonance (NMR).
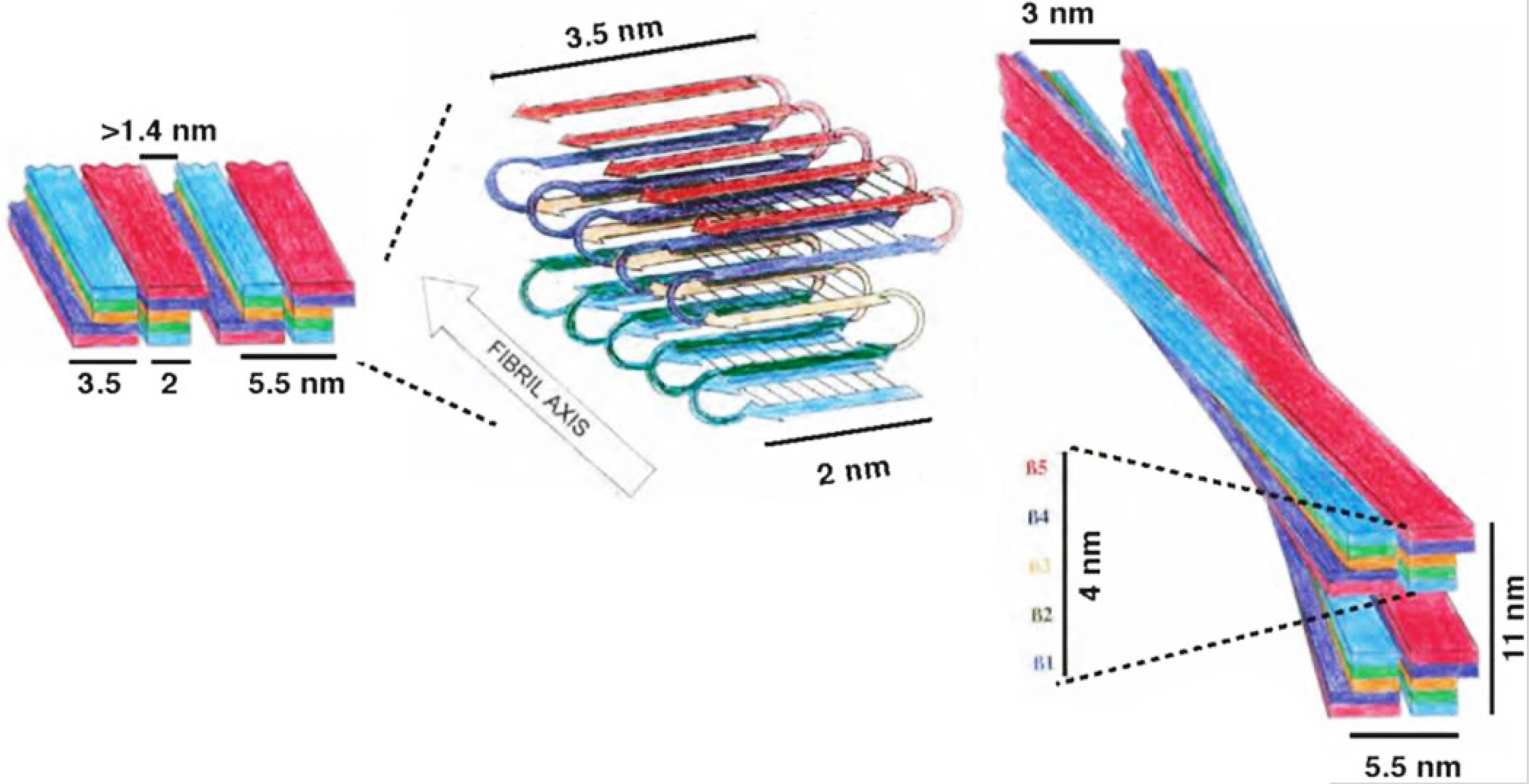
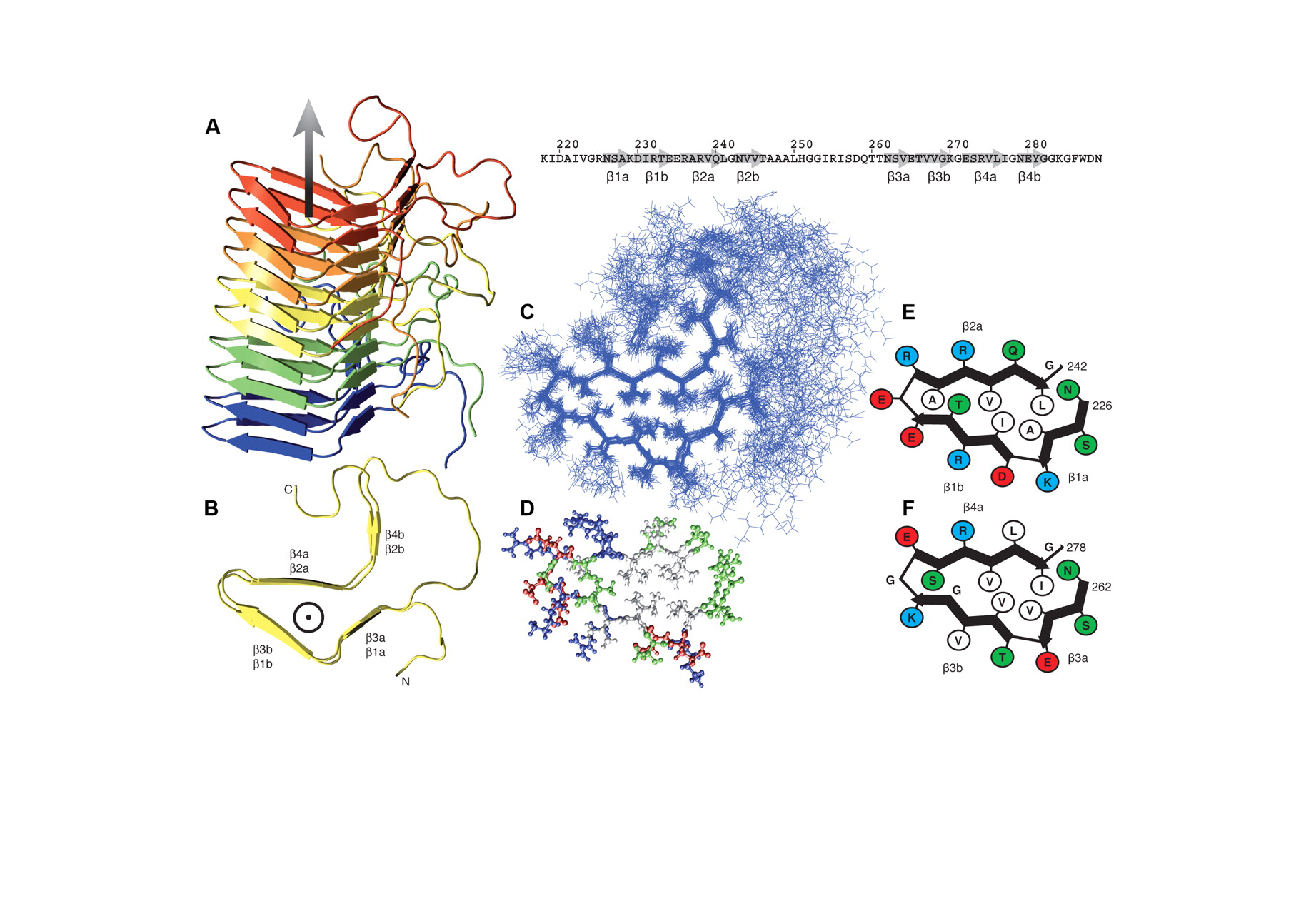
Structure of fibrils:
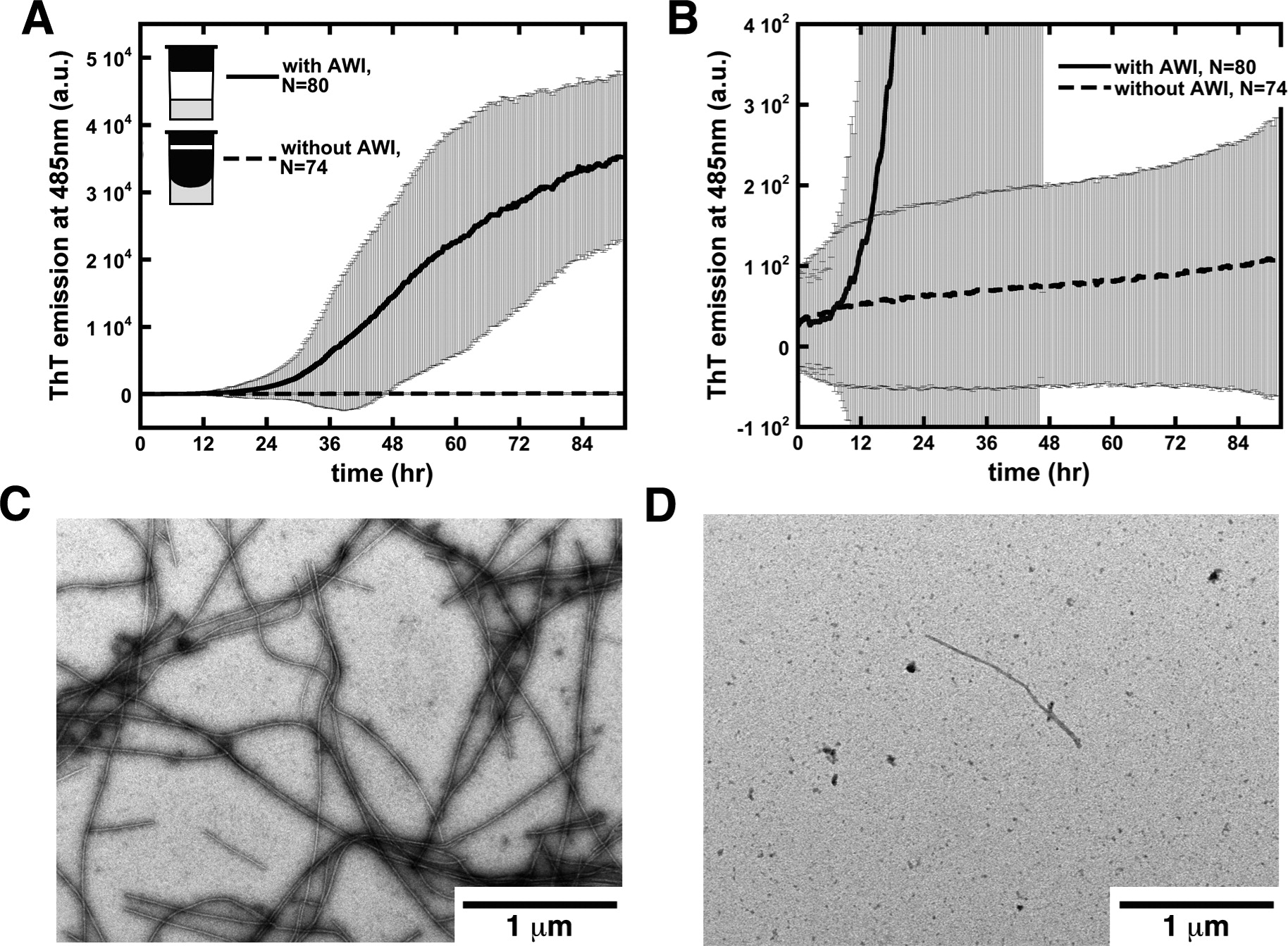
Aggregation Kinetics:
Rate of formation of aggregates, size of aggregates, formation of intermediate aggregates (oligomer and/or proto-fibrils), pathways and morphology of aggregates are found to be dependent on aggregation condition.
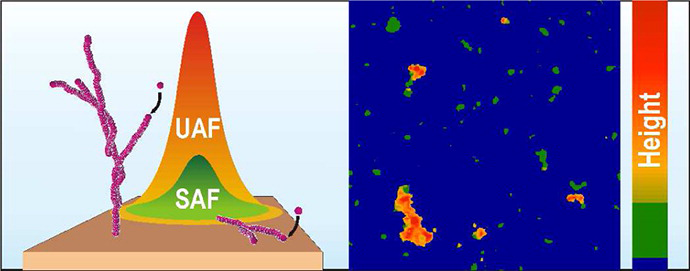
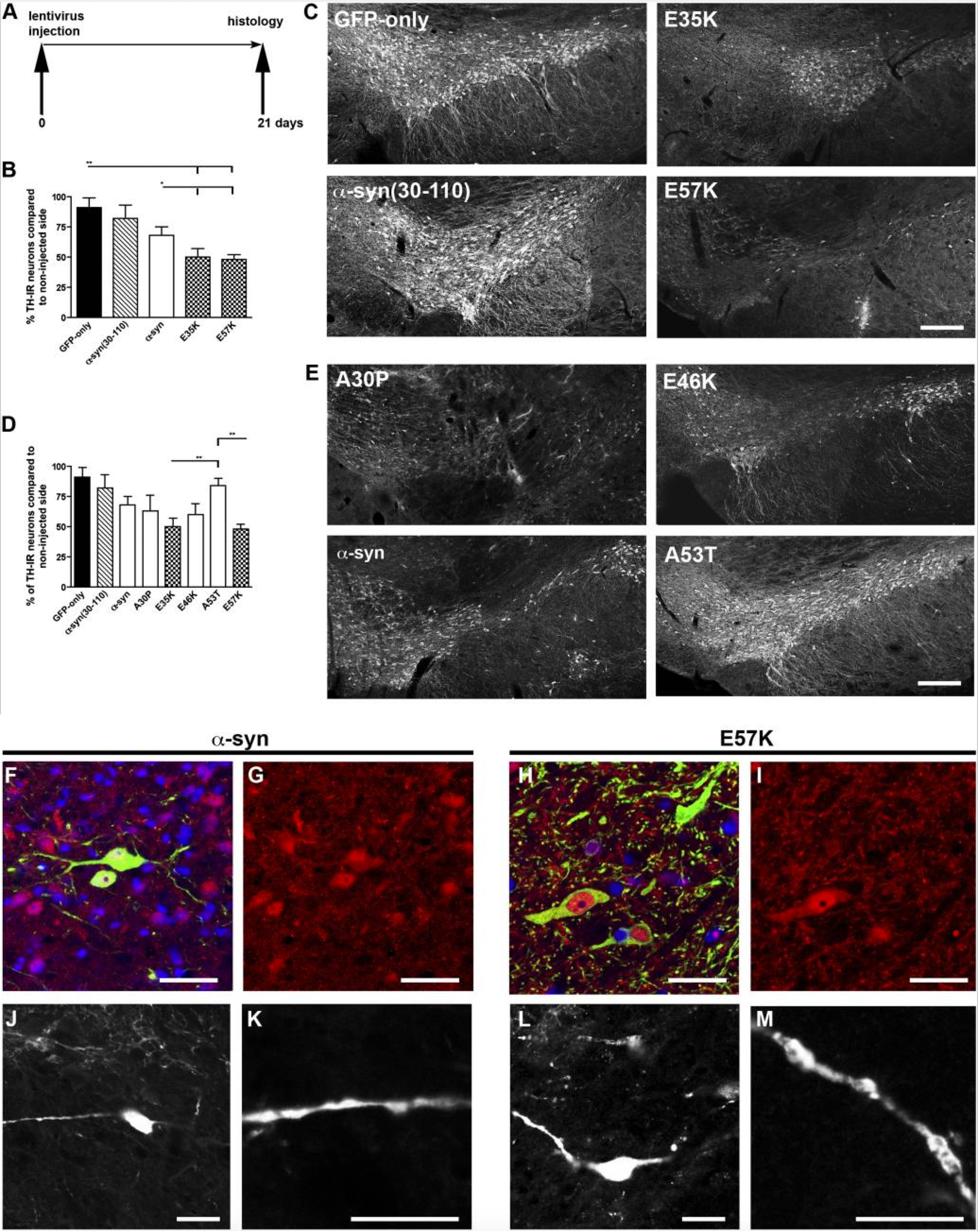
Toxicity of aggregates in mammalian model:
Dopaminergic loss (symptom of Parkinson’s disease) has been monitored in rats. Interestingly variants of alpha synuclein, which form oligomers instead of large aggregates, were found to be the most toxic.