Amyloid Prebiotic Chemistry
Prebiotic Chemistry and the Amyloid World Hypothesis
Investigating the self-organizing and catalytic potential of small peptide aggregates.
By what mechanisms did the first catalytic and replicative molecules come into existence? This and other unanswered (unanswerable?) questions have motivated our lab to investigate the plausibility an “Amyloid World”, that is, a prebiotic earth in which short oligo-peptides aggregated into entities that were able to support the organization, replication and enantio-selectivity that would be required for an early metabolic cycle. In contrast to RNA molecules (the central species in the prevailing “RNA World” hypothesis) peptides in general and amyloids in particular are very stable and could be expected to persist in the harsh conditions of the early earth. Furthermore, amyloids with catalytic functions can be formed from very short peptides (7-mers) whereas RNA appears to require a much longer polymer (50-mer) to attain a catalytic function*.
Amyloids are a unique form of peptide multimer assembly that exist in nature as both functional and aberrant states of proteins. As an ordered aggregregate, the specificity for self-recognition is inherent in the repetitive structure. However, there is some plasticity in the sequence specificity due to the large contribution from the common backbone interactions. Together, the repetitive structure and forgiving sequence specificity provide a window into a protein-like structural landscape for a mixture of short peptides of simple amino acid composition. Thus, amyloid fibrils and other beta-rich aggregates have some interesting implications for the origins of life.
Specifically, we have currently running projects that aim to address the following questions:
What is the catalytic repertoire of peptide amyloid aggregates?
We have developed a method to construct a library of amyloids that can be screened in a high-thoughput manner to search for those peptides, peptide combinations and aggregation conditions that give rise to novel catalytic functions. In our first screen we have identified a set of peptides that catalyze a zinc-dependent hydrolysis of an ester bond and are currently expanding the library size and conditions to search for more efficient catalysts and those with other activities.
To what extent can an amyloid act as a template for the polymerzation of amino acids in a self-replicative manner?
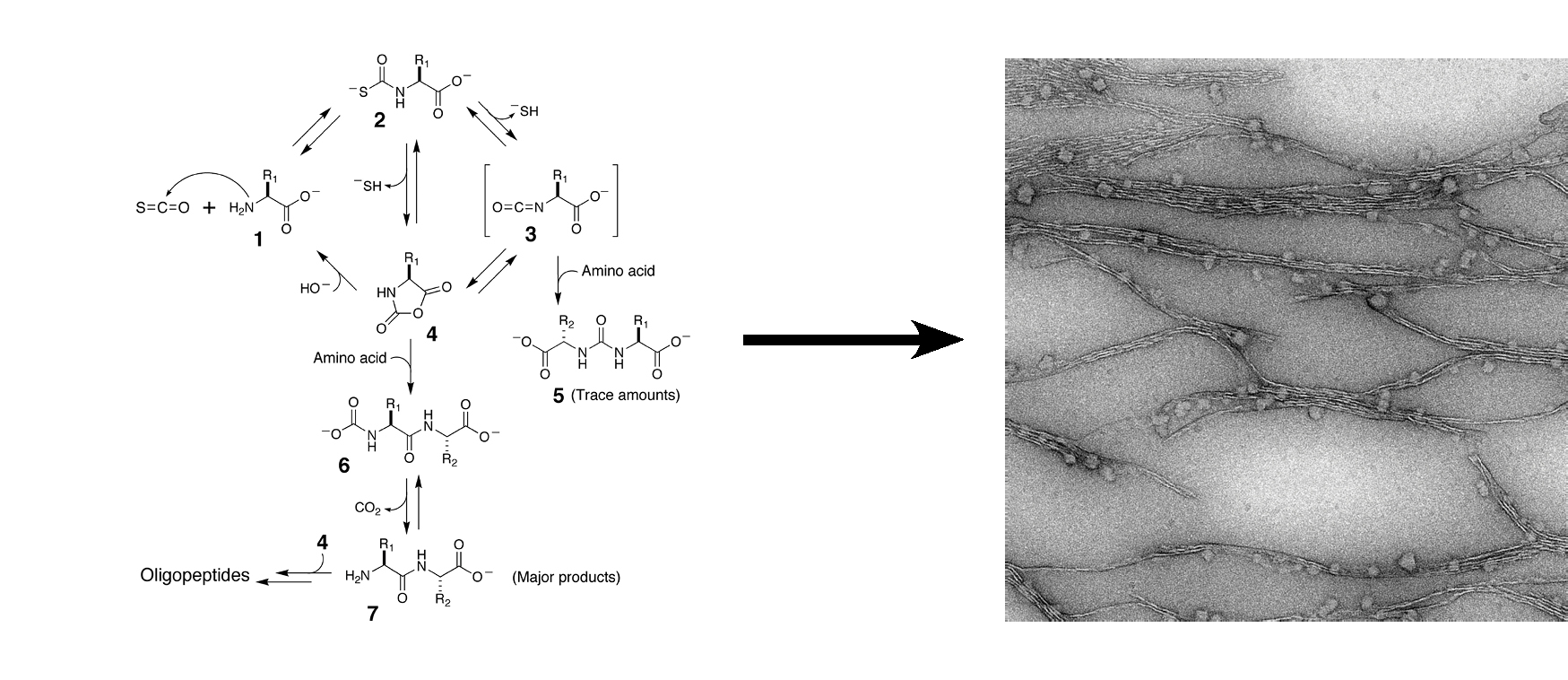
The core structure of the amyloid fold suggests a high level of stereo- and regio-selectivity in the assembly of amyloid aggregates. It had been shown that they can act in a template-like manner in the formation of a covalent bond: in a key experiment the rate at which two fragments of an amyloidogenic peptide condense into the parent peptide is 2-fold higher in the presence of the parent amyloid. While the experimental conditions were non-prebiotic in nature, the researchers show nonetheless that an amyloid can act as a template for the formation of itself from smaller peptide fragments. Motivated by this these results, we want to find ways in which the amyloid can template its own synthesis from simpler components (amino acids) in a more prebiotic setting (using aqueous activating agents that could exist on the early earth). Using a combination of NMR and high-resolution mass spectrometry we analyze the products of complex amino acid polymerization reactions to search for non-random (selective) synthesis of specific peptides.
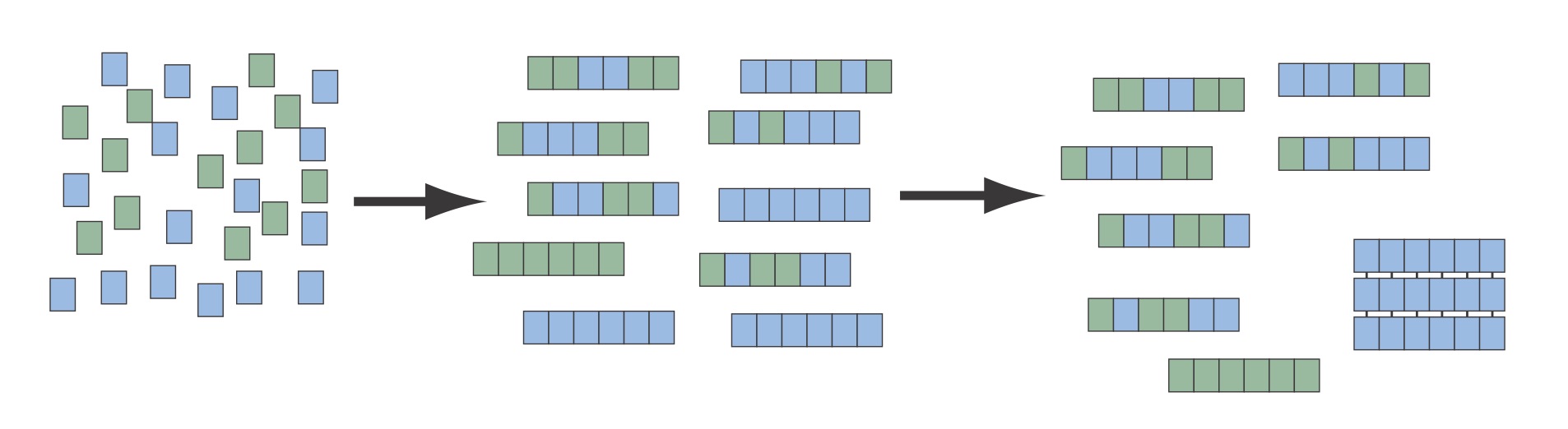
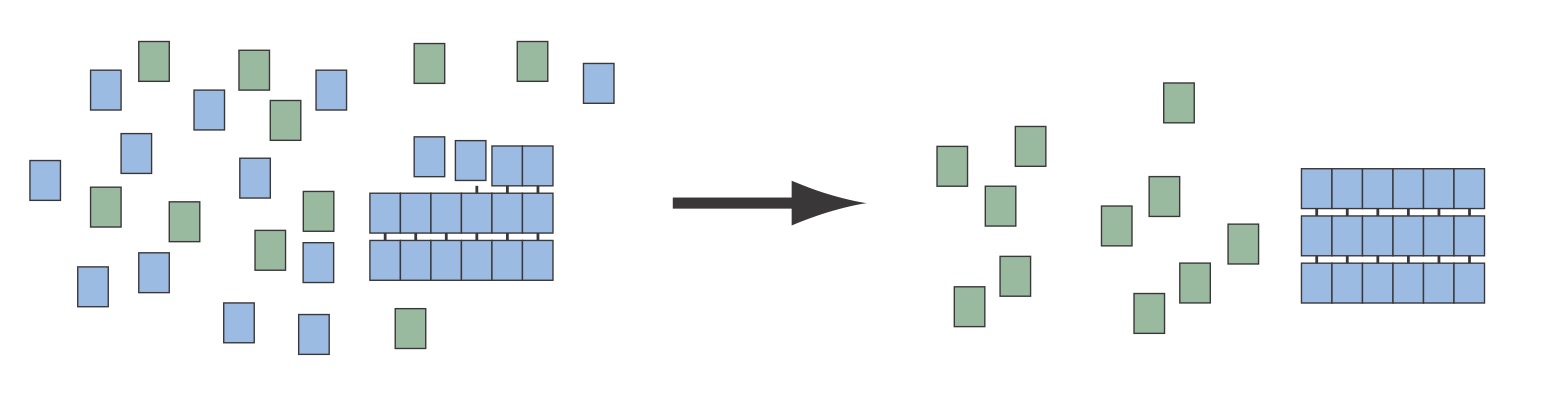
Can an amyloid amplify small enantiomeric excesses to homochirality?
The enantio-specificity of the beta-sheet combined with the insolubility of large amyloid aggregates provide a route to select for homochiral (isotactic) peptides from a mixture of mostly atactic peptides. There is currently no explanation for the rise of homochiral life out of a racemic primitive earth. There are several possible sources of small enantiomeric exceses that could provide some symmetry breaking, but the question remains has to how these small excesses were amplified to homochirality.
*The probability that an RNA molecule, which is sufficiently complex to satisfy the requirements for catalysis, comes into existence via random chemical processes is very small. The probability of more than one identical molecule is essentially nil. The resistance of thephosphodiester backbone of RNA to hydrolysis is also low, making it difficult to imagine sufficient quantities of such molecules surviving long enough in the harsh environment of the early earth to have an impact on chemical evolution.
